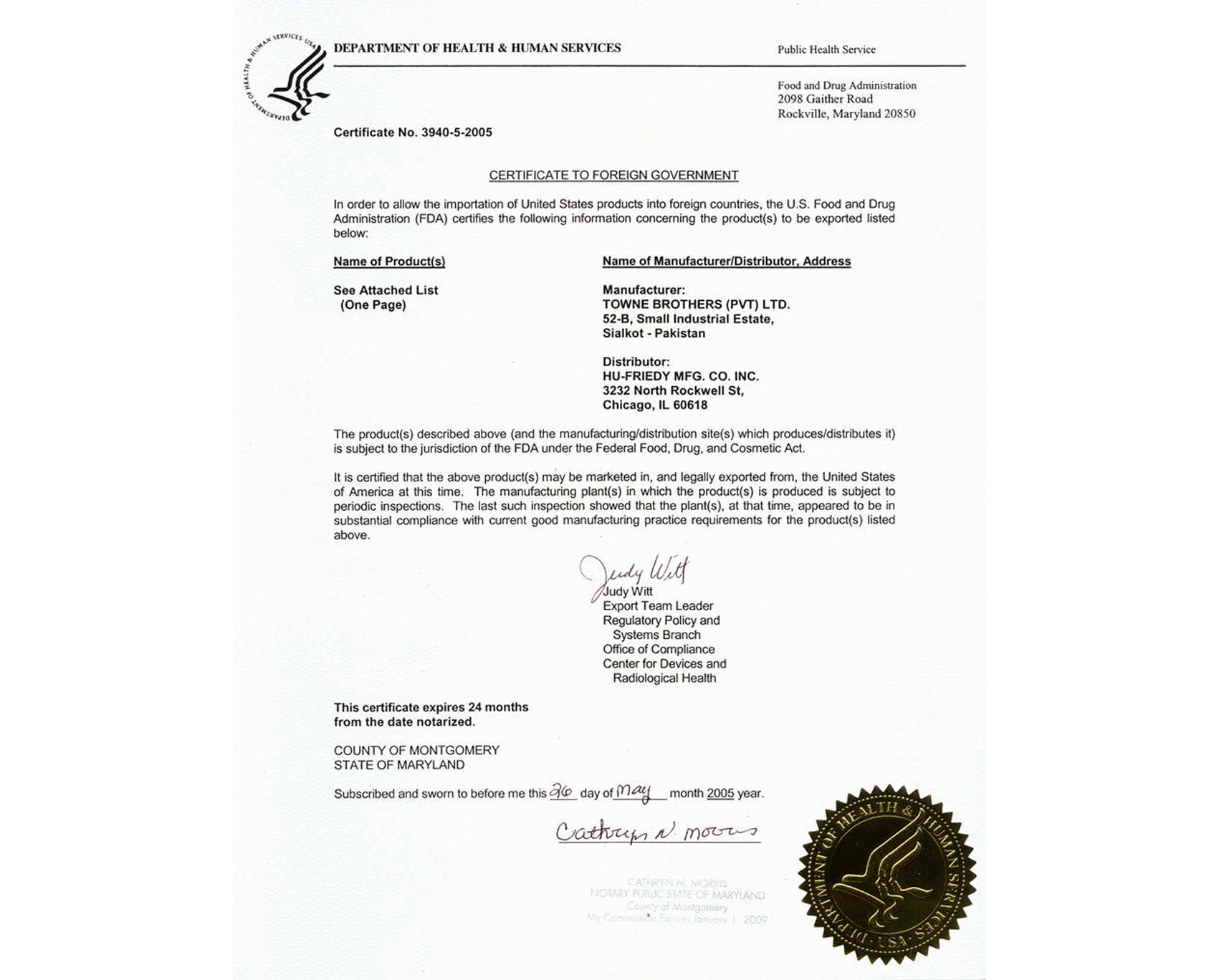
If you intend to export goods to another country, the government that will receive them will ask for a Certificate to Foreign Government, which guarantees that the goods comply with applicable U.S. laws or regulations. The government organization that grants this kind of certification is the Food and Drug Administration (FDA).
Foreign governments frequently request official confirmation that goods supplied to their nations can be sold in the US or comply with particular US requirements, such as the most recent Good Manufacturing Practice (GMP) regulations. To register or import a product into another nation, the FDA Export Certificate may need to be reviewed.
The top provider of document certification, legalization, attestation, and apostille services is Visa Express Inc.
Apostilles may be challenging. Don't trust this technique to untrained workers or unqualified individuals who are unaware of the Apostille procedure and the particular needs of several countries. Your paperwork can be turned down, which would cost you time and money. Be awared!